A coronin7 homolog with functions in actin-driven processes
12-Jan-2010
J. Biol. Chem., in press, doi:10.1074/jbc.M109.083725 published on 12.01.2010
J. Biol. Chem., online article
J. Biol. Chem., online article
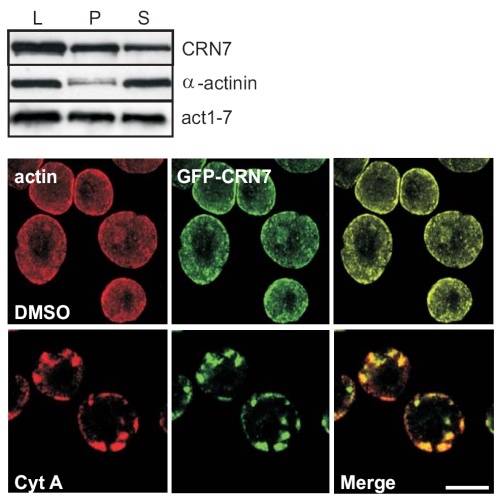
Dictyostelium discoideum Coronin7 (DdCRN7) together with human Coronin7 (CRN7) and Pod-1 of Drosophila melanogaster and Caenorhabditis elegans belong to the coronin family of WD repeat domain containing proteins. Coronin7 proteins are characterized by two WD repeat domains that presumably fold into two beta-propeller structures. DdCRN7 shares highest homology with human CRN7, a protein with roles in membrane trafficking. DdCRN7 is present in the cytosol and accumulates in cell surface projections during movement and phago- and pinocytosis. Cells lacking CRN7 have altered chemotaxis and phagocytosis. Furthermore, loss of CRN7 affects the infection process by the pathogen Legionella pneumophila and allows a more efficient internalization of bacteria. To provide a mechanism for CNR7 action we studied actin related aspects. We could show that CRN7 binds directly to F-actin and protects actin filaments from depolymerization. CRN7 also associated with F-actin in vivo. It was present in the Triton X-100 insoluble cytoskeleton, colocalized with F-actin and its distribution was sensitive to drugs affecting the actin cytoskeleton. We propose that CRN7's role in chemotaxis and phagocytosis is through its effect on the actin cytoskeleton.