A Minimal β-Lactone Fragment for Selective β5c or β5i Proteasome Inhibitors
14-May-2015
Angewandte Chemie, 2015, DOI: 10.1002/anie.201502931, Volume 54, Issue 27, pages 7810–7814, published on 14.05.2015
Angewandte Chemie, online article
Angewandte Chemie, online article
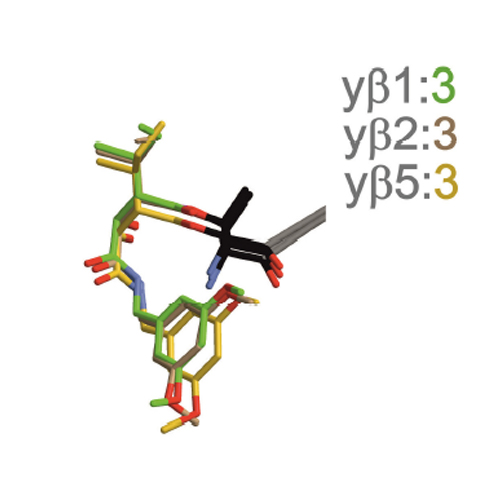
Broad-spectrum proteasome inhibitors are applied as anticancer drugs, whereas selective blockage of the immunoproteasome represents a promising therapeutic rationale for autoimmune diseases. We here aimed at identifying minimal structural elements that confer β5c or β5i selectivity on proteasome inhibitors. Based on the natural product belactosin C, we synthesized two β-lactones featuring a dimethoxybenzyl moiety and either a methylpropyl (pseudo-isoleucin) or an isopropyl (pseudo-valine) P1 side chain. Although the two compounds differ only by one methyl group, the isoleucine analogue is six times more potent for β5i (IC50=14 nM) than the valine counterpart. Cell culture experiments demonstrate the cell-permeability of the compounds and X-ray crystallography data highlight them as minimal fragments that occupy primed and non-primed pockets of the active sites of the proteasome. Together, these results qualify β-lactones as a promising lead-structure motif for potent nonpeptidic proteasome inhibitors with diverse pharmaceutical applications.