A single residue switch reveals principles of antibody domain integrity
18-Sep-2018
J. Biol. Chem. (2018) 293(44) 17107–17118, DOI 10.1074/jbc.RA118.005475
J. Biol. Chem., online article
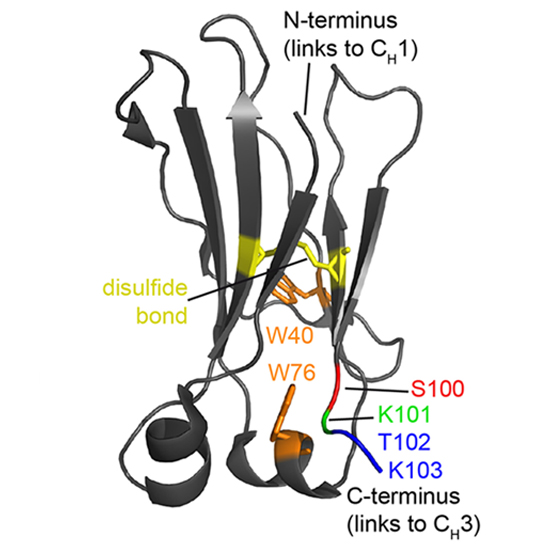
Despite their importance for antibody architecture and design, the principles governing antibody domain stability are still not understood in sufficient detail. Here, to address this question, we chose a domain from the invariant part of IgG, the CH2 domain. We found that compared with other Ig domains, the isolated CH2 domain is a surprisingly unstable monomer, exhibiting a melting temperature of ~ 44 °C. We further show that the presence of an additional C-terminal lysine in a CH2 variant substantially increases the melting temperature by ~ 14 °C relative to CH2 wt. To explore the molecular mechanism of this effect, we employed biophysical approaches to probe structural features of CH2. The results revealed that Lys101 is key for the formation of three secondary structure elements: the very C-terminal β-strand and two adjacent α-helices. We also noted that a dipole interaction between Lys101 and the nearby α-helix, is important for stabilizing the CH2 architecture by protecting the hydrophobic core. Interestingly, this interaction between the α-helix and C-terminal charged residues is highly conserved in antibody domains, suggesting that it represents a general mechanism for maintaining their integrity. We conclude that the observed interactions involving terminal residues have practical applications for defining domain boundaries in the development of antibody therapeutics and diagnostics.