An Unfolded CH1 Domain Controls the Assembly and Secretion of IgG Antibodies
12-Jun-2009
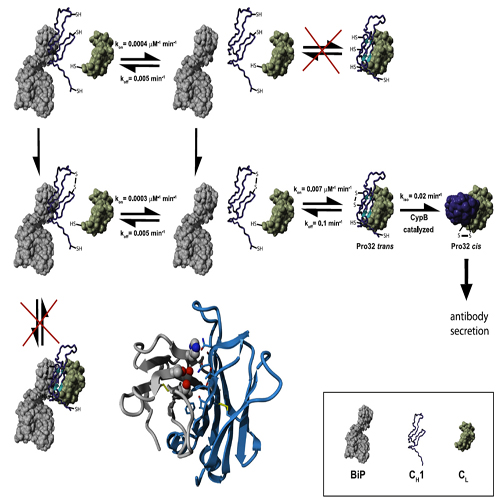
A prerequisite for antibody secretion and function is their assembly into a defined quaternary structure, composed of two heavy and two light chains for IgG. Unassembled heavy chains are actively retained in the endoplasmic reticulum (ER). Here, we show that the CH1 domain of the heavy chain is intrinsically disordered invitro, which sets it apart from other antibody domains. It folds only upon interaction with the light-chain CL domain. Structure formation proceeds via a trapped intermediate and can be accelerated by the ER-specific peptidyl-prolyl isomerase cyclophilin B. The molecular chaperone BiP recognizes incompletely folded states of the CH1 domain and competes for binding to the CL domain. In vivo experiments demonstrate that requirements identified for folding the CH1 domain invitro, including association with a folded CL domain and isomerization of a conserved proline residue, are essential for antibody assembly and secretion in the cell.