Chaperonin-Catalyzed Rescue of Kinetically Trapped States in Protein Folding
09-Jul-2010
Cell, 2010, doi:10.1016/j.cell.2010.05.027, Volume 142, Issue 1, 112-122 published on 09.07.2010
Cell, online article
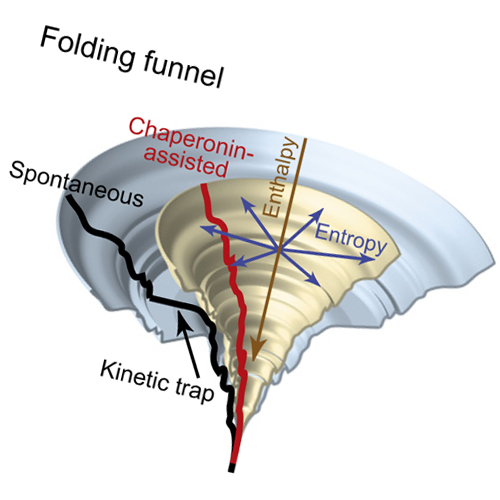
GroEL and GroES form a chaperonin nano-cage for single protein molecules to fold in isolation. The folding properties that render a protein chaperonin dependent are not yet understood. Here, we address this question using a double mutant of the maltosebinding protein DM-MBP as a substrate. Upon spontaneous refolding, DM-MBP populates a kinetically trapped intermediate that is collapsed but structurally disordered. Introducing two long-range disulfide bonds into DM-MBP reduces the entropic folding barrier of this intermediate and strongly accelerates native state formation. Strikingly, steric confinement of the protein in the chaperonin cage mimics the kinetic effect of constraining disulfides on folding, in a manner mediated by negative charge clusters in the cage wall. These findings suggest that chaperonin dependence correlates with the tendency of proteins to populate entropically stabilized folding intermediates. The capacity to rescue proteins from such folding traps may explain the uniquely essential role of chaperonin cages within the cellular chaperone network.