Distinct roles of the two isoforms of the dynamin-like GTPase Mgm1 in mitochondrial fusion
07-Jul-2009
FEBS Lett., 2009, 583(13), 2237-2243, doi:10.1016/j.febslet.2009.05.053 published on 07.07.2009
FEBS Letters, online article
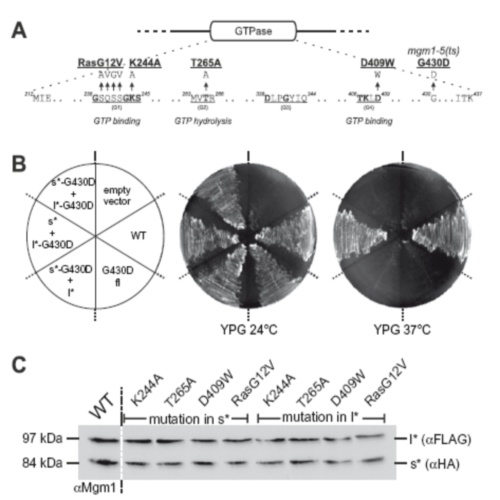
The mitochondrial dynamin-like GTPase Mgm1 exists as a long (l-Mgm1) and a short isoform (s-Mgm1). They both are essential for mitochondrial fusion. Here we show that the isoforms interact in a homotypic and heterotypic manner. Their submitochondrial distribution between inner boundary membrane and cristae was markedly different. Overexpression of l-Mgm1 exerts a dominant negative effect on mitochondrial fusion. A functional GTPase domain is required only in s-Mgm1 but not in l-Mgm1. We propose that l-Mgm1 acts primarily as an anchor in the inner membrane that in concert with the GTPase activity of s-Mgm1 mediates the fusion of inner membranes.