Local conformational dynamics in alpha-helices measured by fast triplet transfer
08-Jan-2009
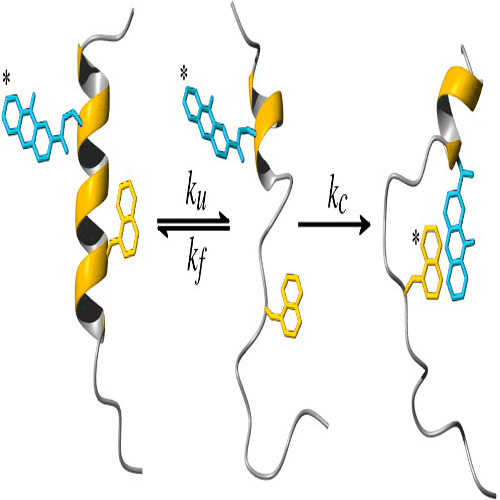
Coupling fast triplet–triplet energy transfer (TTET) between xanthone and naphthylalanine to the helix–coil equilibrium in alanine-based peptides allowed the observation of local equilibrium fluctuations in α-helices on the nanoseconds to microseconds time scale. The experiments revealed faster helix unfolding in the terminal regions compared with the central parts of the helix with time constants varying from 250 ns to 1.4 μs at 5 °C. Local helix formation occurs with a time constant of ≈400 ns, independent of the position in the helix. Comparing the experimental data with simulations using a kinetic Ising model showed that the experimentally observed dynamics can be explained by a 1-dimensional boundary diffusion with position-independent elementary time constants of ≈50 ns for the addition and of ≈65 ns for the removal of an α-helical segment. The elementary time constant for helix growth agrees well with previously measured time constants for formation of short loops in unfolded polypeptide chains, suggesting that helix elongation is mainly limited by a conformational search.