Mapping backbone and side-chain interactions in the transition state of a coupled protein folding and binding reaction
16-Feb-2011
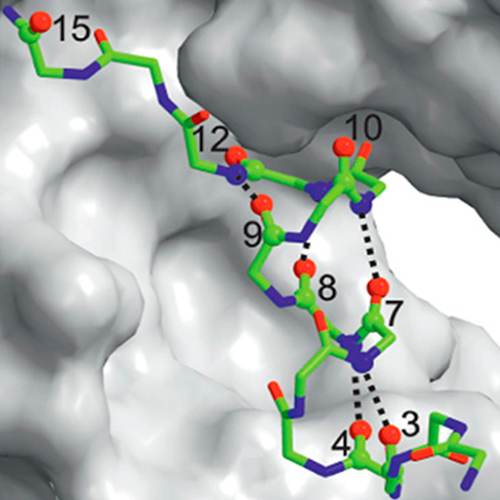
Understanding the mechanism of protein folding requires a detailed knowledge of the structural properties of the barriers separating unfolded from native conformations. The S-peptide from ribonuclease S forms its Alpha-helical structure only upon binding to the folded S-protein. We characterized the transition state for this binding-induced folding reaction at high resolution by determining the effect of site-specific backbone thioxylation and side-chainmodifications on the kinetics and thermodynamics of the reaction, which allows us to monitor formation of backbone hydrogen bonds and side-chain interactions in the transition state. The experiments reveal that Alpha-helical structure in the S-peptide is absent in the transition state of binding. Recognition between the unfolded S-peptide and the S-protein is mediated by loosely packed hydrophobic side-chain interactions in two well defined regions on the S-peptide. Close packing and helix formation occurs rapidly after binding. Introducing hydrophobic residues at positions outside the recognition region can drastically slow down association.