Modulation of Small GTPases by Legionella
05-Aug-2013
Curr Top Microbiol Immunol., 2013, DOI: 10.1007/82_2013_340, 376: 117–133 published on 06.08.2013
Curr Top Microbiol Immunol., online article
Curr Top Microbiol Immunol., online article
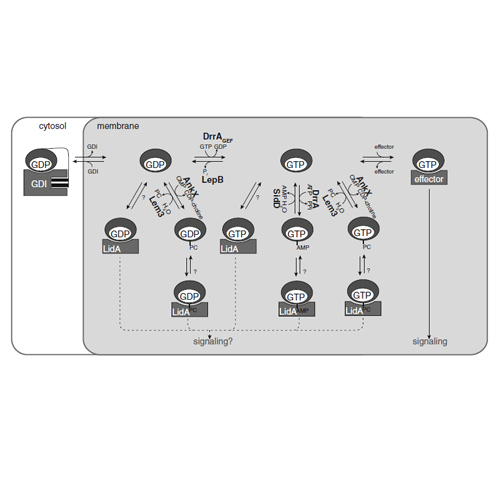
The pathogenic bacterium Legionella pneumophila interacts intimately with signaling molecules during the infection of eukaryotic host cells. Among a diverse set of regulatory molecules, host small GTPases appear to be prominent and significant targets. Small GTPases are molecular switches that regulate cellular signaling via their respective nucleotide-bound states: When bound to GDP, they are inactive, but become activated upon binding to GTP. Legionella secretes specific bacterial proteins into the cytosol of the host cell that most prominently modulate the activities of small GTPases involved in vesicular trafficking, but probably also other G-proteins. The master regulators of vesicular trafficking, i.e., Rab and Arf proteins, are majorly targeted G-proteins of Legionella proteins, and among these, Rab1 experiences the most diverse modifications. Generally, the activities of small GTPases are modulated by GDP/GTP exchange (activation), GTP hydrolysis (deactivation), membrane recruitment, post-translational modifications (phosphocholination, adenylylation), and tight and competitive binding. Here, we discuss the consequences and molecular details of the modulation of small GTPases for the infection by Legionella, with a special but not exclusive focus on Rab and Arf proteins.