Phosphorylation of Arabidopsis transketolase at Ser428 provides a potential paradigm for the metabolic control of chloroplast carbon metabolism
01-Feb-2014
Biochem. J., 2014, doi:10.1042/BJ20130631, 313–322 published on 01.02.2014
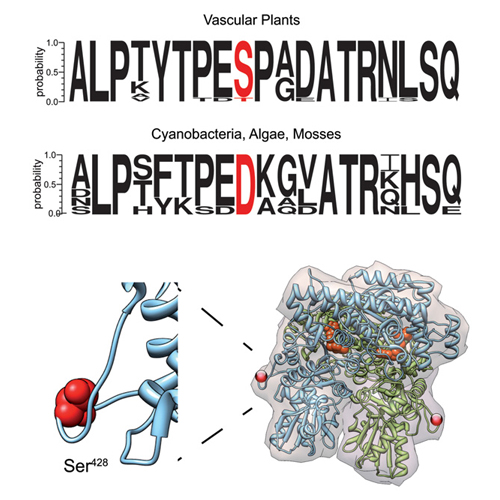
Calcium is an important second messenger in eukaryotic cells that regulates many different cellular processes. To elucidate calcium regulation in chloroplasts, we identified the targets of calcium-dependent phosphorylation within the stromal proteome. A 73 kDa protein was identified as one of the most dominant proteins undergoing phosphorylation in a calcium-dependent manner in the stromal extracts of both Arabidopsis and Pisum. It was identified as TKL (transketolase), an essential enzyme of both the Calvin–Benson–Bassham cycle and the oxidative pentose phosphate pathway. Calcium-dependent phosphorylation of both Arabidopsis isoforms (AtTKL1 and AtTKL2) could be confirmed in vitro using recombinant proteins. The phosphorylation is catalysed by a stroma-localized protein kinase, which cannot utilize GTP. Phosphorylation of AtTKL1, the dominant isoform in most tissues, occurs at a serine residue that is conserved in TKLs of vascular plants. By contrast, an aspartate residue is present in this position in cyanobacteria, algae and mosses. Characterization of a phosphomimetic mutant (S428D) indicated that Ser428 phosphorylation exerts significant effects on the enzyme's substrate saturation kinetics at specific physiological pH values. The results of the present study point to a role for TKL phosphorylation in the regulation of carbon allocation.