Principles and engineering of antibody folding and assembly
13-Jun-2014
Biochimica et Biophysica Acta, 2014, doi:10.1016/j.bbapap.2014.06.004, Volume 1844, Issue 11, Pages 2024–2031 published on 13.06.2014
Biochimica et Biophysica Acta, online article
Biochimica et Biophysica Acta, online article
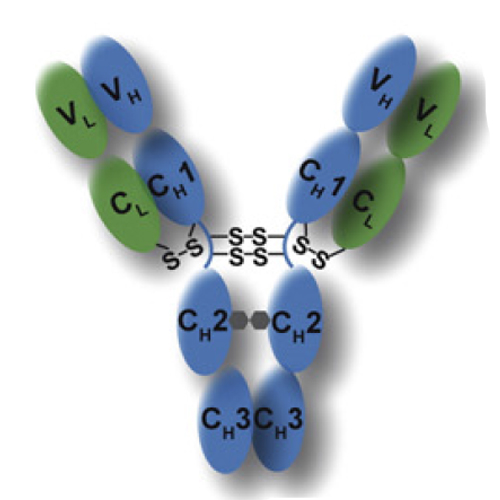
Antibodies are uniquely suited to serve essential roles in the human immune defense as they combine several specific functions in one hetero-oligomeric protein. Their constant regions activate effector functions and their variable domains provide a stable framework that allows incorporation of highly diverse loop sequences. The combination of non-germline DNA recombination and mutation together with heavy and light chain assembly allows developing variable regions that specifically recognize essentially any antigen they may encounter. However, this diversity also requires tailor-made mechanisms to guarantee that folding and association of antibodies is carefully this diversity also requires tailor-made mechanisms to guarantee that folding and association of antibodies is carefully controlled before the protein is secreted from a plasma cell. Accordingly, the generic immunoglobulin fold β-barrel structure of antibody domains has been fine-tuned during evolution to fit the different requirements. Work over the past decades has identified important aspects of the folding and assembly of antibody domains and chains revealing domain specific variations of a general scheme. The most striking is the folding of an intrinsically disordered antibody domain in the context of its partner domain as the basis for antibody assembly and its control on the molecular level in the cell. These insights have not only allowed a better understanding of the antibody folding process but also provide a wealth of opportunities for rational optimization of antibody molecules. In this review, we summarize current concepts of antibody folding and assembly and discuss how they can be utilized to engineer antibodies with improved performance for different applications. This article is part of a Special Issue entitled: Recent advances in the molecular engineering of antibodies.