Protein disulfide isomerase isomerizes non-native disulfide bonds in human proinsulin independent of its peptidebinding activity
24-Jan-2011
Protein Science, 2011, DOI: 10.1002/pro.592, VOL 20:588—596 published on 24.01.2011
Protein Science, online article
Protein Science, online article
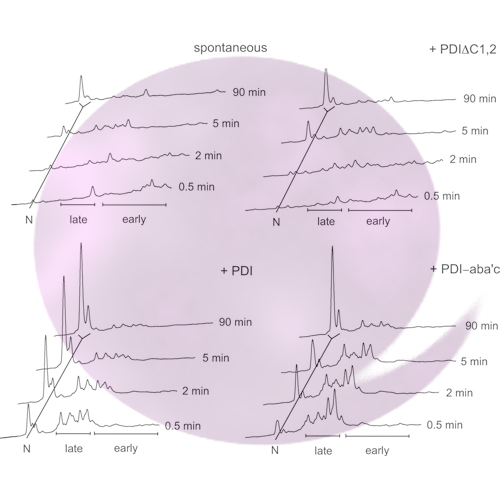
Protein disulfide isomerase (PDI) supports proinsulin folding as chaperone and isomerase. Here, we focus on how the two PDI functions influence individual steps in the complex folding process of proinsulin. We generated a PDI mutant (PDI‐aba′c) where the b′ domain was partially deleted, thus abolishing peptide binding but maintaining a PDI‐like redox potential. PDI‐aba′c catalyzes the folding of human proinsulin by increasing the rate of formation and the final yield of native proinsulin. Importantly, PDI‐aba′c isomerizes non‐native disulfide bonds in completely oxidized folding intermediates, thereby accelerating the formation of native disulfide bonds. We conclude that peptide binding to PDI is not essential for disulfide isomerization in fully oxidized proinsulin folding intermediates.