Pseudilins: Halogenated, Allosteric Inhibitors of the Non-Mevalonate Pathway Enzyme IspD
20-Jan-2014
Angewandte Chemie, 2014, DOI: 10.1002/anie.201309557, Volume 53, Issue 8, pages 2235–2239, published on 20.01.2014
Angewandte Chemie, online article
Angewandte Chemie, online article
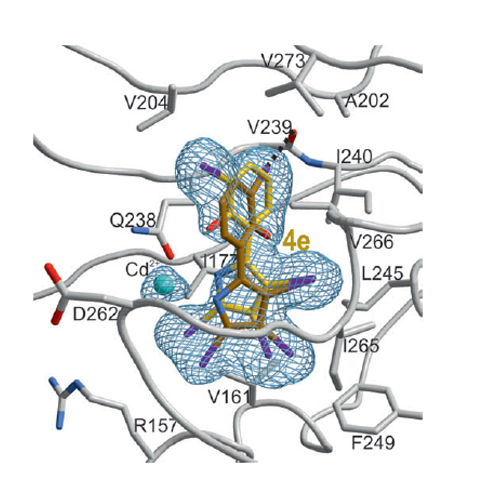
The enzymes of the non-mevalonate pathway for isoprenoid biosynthesis have been identified as attractive targets with novel modes of action for the development of herbicides for crop protection and agents against infectious diseases. This pathway is present in many pathogenic organisms and plants, but absent in mammals. By using high-throughput screening, we identified highly halogenated marine natural products, the pseudilins, to be inhibitors of the third enzyme, IspD, in the pathway. Their activity against the IspD enzymes from Arabidopsis thaliana and Plasmodium vivax was determined in photometric and NMR-based assays. Cocrystal structures revealed that pseudilins bind to an allosteric pocket by using both divalent metal ion coordination and halogen bonding. The allosteric mode of action for preventing cosubstrate (CTP) binding at the active site was elucidated. Pseudilins show herbicidal activity in plant assays and antiplasmodial activity in cell-based assays.