Receptor-Bound Conformation of Cilengitide Better Represented by Its Solution-State Structure than the Solid-State Structure
22-Sep-2014
Chemistry - A European Journal, 2014, DOI: 10.1002/chem.201403839, Volume 20, Issue 44, pages 14201–14206, published on 22.09.2014
Chemistry - A European Journal, online article
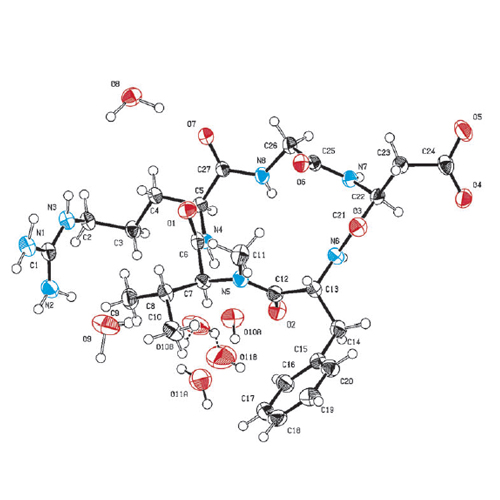
The X-ray crystal and NMR spectroscopic structures of the peptide drug candidate Cilengitide (cyclo(RGDf(NMe)Val)) in various solvents are obtained and compared in addition to the integrin receptor bound conformation. The NMR-based solution structures exhibit conformations closely resembling the X-ray structure of Cilengitide bound to the head group of integrin αvβ3. In contrast, the structure of pure Cilengitide recrystallized from methanol reveals a different conformation controlled by the lattice forces of the crystal packing. Molecular modeling studies of the various ligand structures docked to the αvβ3 integrin revealed that utilization of the solid-state conformation of Cilengitide leads—unlike the solution-based structures—to a mismatch of the ligand–receptor interactions compared with the experimentally determined structure of the protein–ligand complex. Such discrepancies between solution and crystal conformations of ligands can be misleading during the structure-based lead optimization process and should thus be taken carefully into account in ligand orientated drug design.