Regulated structural transitions unleash the chaperone activity of αB-crystallin
01-Oct-2013
PNAS, 2013, doi: 10.1073/pnas.1308898110, vol. 110 no. 40 E3780-E3789 published on 01.10.2013
PNAS, online article
PNAS, online article
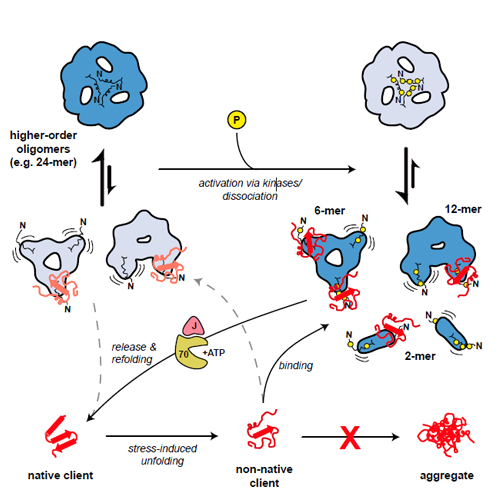
The small heat shock protein αB-crystallin is an oligomeric molecular chaperone that binds aggregation-prone proteins. As a component of the proteostasis system, it is associated with cataract, neurodegenerative diseases, and myopathies. The structural determinants for the regulation of its chaperone function are still largely elusive. Combining different experimental approaches, we show that phosphorylation-induced destabilization of intersubunit interactions mediated by the N-terminal domain (NTD) results in the remodeling of the oligomer ensemble with an increase in smaller, activated species, predominantly 12-mers and 6-mers. Their 3D structures determined by cryo-electron microscopy and biochemical analyses reveal that the NTD in these species gains flexibility and solvent accessibility. These modulated properties are accompanied by an increase in chaperone activity in vivo and in vitro and a more efficient cooperation with the heat shock protein 70 system in client folding. Thus, the modulation of the structural flexibility of the NTD, as described here for phosphorylation, appears to regulate the chaperone activity of αB-crystallin rendering the NTD a conformational sensor for nonnative proteins.