Semisynthesis of a Glycosylphosphatidylinositol-Anchored Prion Protein
13-Oct-2008
Angewandte Chemie, 2008, DOI: 10.1002/anie.200802161, Volume 47, Issue 43, pages 8215–8219, published on 13.10.2008
Angew. Chemie, online article
Angew. Chemie, online article
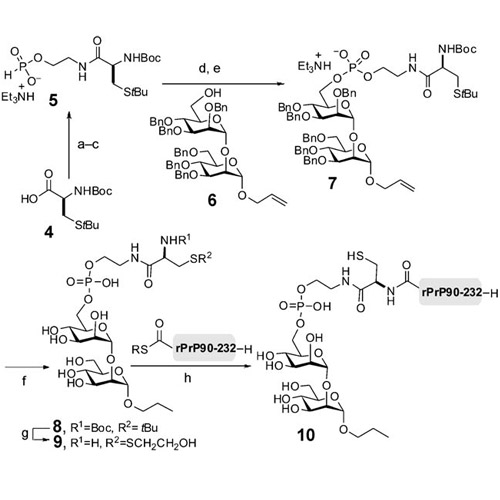
Proteins are often modified posttranslationally by glycosylation and lipidation. Glycosylphosphatidylinositol (GPI) anchors combine both types of modification and link many proteins to the cell surface. Advances in solid-phase peptide synthesis (SPPS) and recombinant protein engineering, in combination with the development of native chemical ligation (NCL) and expressed protein ligation (EPL), have resulted in numerous total syntheses and semisyntheses of proteins. These approaches facilitate access to homogeneous glycoand lipoproteins, which serve as defined molecular probes to elucidate the effects of glycosylation and lipidation on the biophysical properties of proteins. Synthetic GPI glycans and lipidated GPI anchors have emerged as valuable tools that allow for the precise dissection of their biological relevance in infectious and metabolic diseases. Efforts towards the assembly of chemically defined GPI-anchored proteins have focused on model studies; no synthetic GPIanchored protein has been reported to date.