Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53
04-Sep-2011
Nature Structural & Molecular Biology, 2011, doi:10.1038/nsmb.2114, published on 04.09.2011
Nature Structural & Molecular Biology, online article
Nature Structural & Molecular Biology, online article
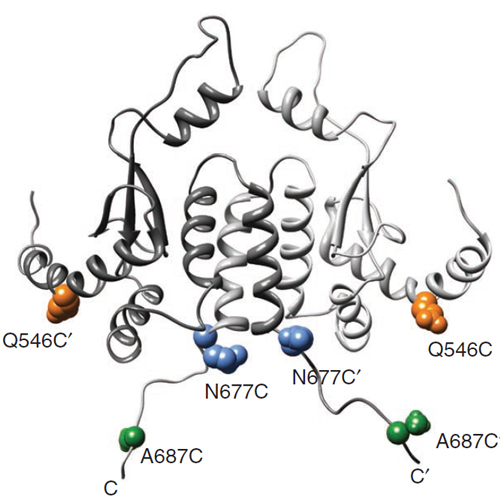
In eukaryotes, the essential dimeric molecular chaperone Hsp90 is required for the activation and maturation of specific substrates such as steroid hormone receptors, tyrosine kinases and transcription factors. Hsp90 is involved in the establishment of cancer and has become an attractive target for drug design. Here we present a structural characterization of the complex between Hsp90 and the tumor suppressor p53, a key mediator of apoptosis whose structural integrity is crucial for cell-cycle control. Using biophysical methods, we show that the human p53 DNA-binding domain interacts with multiple domains of yeast Hsp90. p53 binds to the Hsp90 C-terminal domain in its native-like state in a charge-dependent manner, but it also associates weakly with binding sites in the middle and the N-terminal domains. The fine-tuned interplay between several Hsp90 domains provides the interactions required for efficient chaperoning of p53.