Structural and Mechanical Hierarchies in the Alpha Crystallin Domain Dimer of the Hyperthermophilic Small Heat Shock Protein Hsp16.5
30-Jul-2010
Journal of Molecular Biology, 2010, doi:10.1016/j.jmb.2010.05.065, Volume 400, Issue 5, Pages 1046-1056 published on 30.07.2010
Journal of Molecular Biology, online article
Journal of Molecular Biology, online article
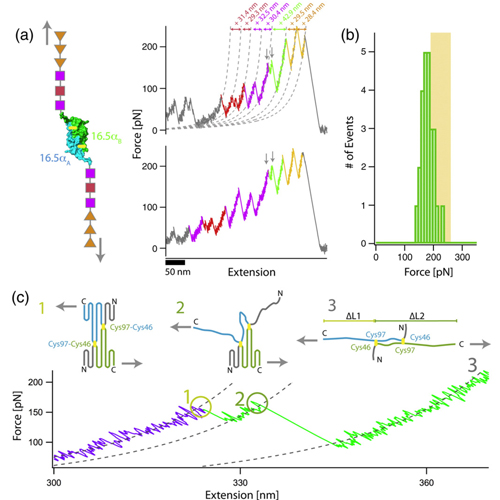
In biological systems, proteins rarely act as isolated monomers. Association to dimers or higher oligomers is a commonly observed phenomenon. As an example, small heat shock proteins form spherical homo-oligomers of mostly 24 subunits, with the dimeric αlpha-crystallin domain as the basic structural unit. The structural hierarchy of this complex is key to its function as a molecular chaperone. In this article, we analyze the folding and association of the basic building block, the αlpha-crystallin domain dimer, from the hyperthermophilic archaeon Methanocaldococcus jannaschii Hsp16.5 in detail. Equilibrium denaturation experiments reveal that the αlpha-crystallin domain dimer is highly stable against chemical denaturation. In these experiments, protein dissociation and unfolding appear to follow an “all-ornone” mechanism with no intermediate monomeric species populated. When the mechanical stability was determined by single-molecule force spectroscopy, we found that the αlpha-crystallin domain dimer resists high forces when pulled at its termini. In contrast to bulk denaturation, stable monomeric unfolding intermediates could be directly observed in the mechanical unfolding traces after the αlpha-crystallin domain dimer had been dissociated by force. Our results imply that for this hyperthermophilic member of the small heat shock protein family, assembly of the spherical 24mer starts from folded monomers, which readily associate to the dimeric structure required for assembly of the higher oligomer.