Substrate discrimination of the chaperone BiP by autonomous and cochaperone-regulated conformational transitions
09-Jan-2011
Nature Strucrural & Molecular Biology,, 2011, doi:10.1038/nsmb.1970, Pages:150–158 (2011) published on 09.01.2011
Nature Strucrural & Molecular Biology, online article
Nature Strucrural & Molecular Biology, online article
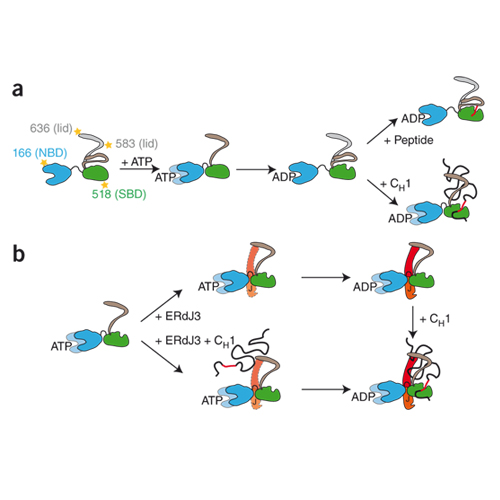
The endoplasmic reticulum is the site of folding, assembly and quality control for proteins of the secretory pathway. The ATP-regulated Hsp70 chaperone BiP (heavy chain–binding protein), together with cochaperones, has important roles in all of these processes. The functional cycle of Hsp70s is determined by conformational transitions that are required for substrate binding and release. Here, we used the intrinsically disordered CH1 1 domain of antibodies as an authentic substrate protein and analyzed the conformational cycle of BiP by single-molecule and ensemble Förster resonance energy transfer (FRET) measurements. Nucleotide binding resulted in concerted domain movements of BiP. Conformational transitions of the lid domain allowed BiP to discriminate between peptide and protein substrates. A major BiP cochaperone in antibody folding, ERdj3, modulated the conformational space of BiP in a nucleotide-dependent manner, placing the lid subdomain in an open, protein-accepting state.