The Chaperone Activity and Substrate Spectrum of Human Small Heat Shock Proteins
30-Nov-2016
THE JOURNAL OF BIOLOGICAL CHEMISTRY, VOL. 292, NO. 2, pp. 672–684, DOI 10.1074/jbc.M116.760413
THE JOURNAL OF BIOLOGICAL CHEMISTRY, online article
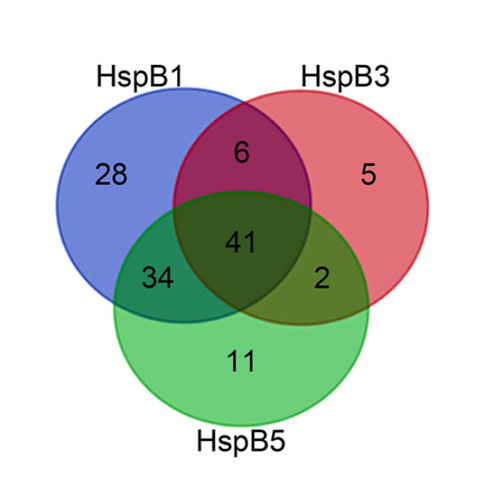
Small heat shock proteins (sHsps) are a ubiquitous family of molecular chaperones that suppress the unspecific aggregation of miscellaneous proteins. Multicellular organisms contain a large number of different sHsps, raising questions as to whether they function redundantly or are specialized in terms of substrates and mechanism. To gain insight into this issue, we undertook a comparative analysis of the 8 major human sHsps on the aggregation of both model proteins and cytosolic lysates under standardized conditions. We discovered that sHsps which form large oligomers (HspB1/Hsp27, HspB3, HspB4/αA-crystallin, and HspB5/αB-crystallin) are promiscuous chaperones, whereas the chaperone activity of the other sHsps is more substrate-dependent. However, all human sHsps analyzed, except HspB7, suppressed the aggregation of cytosolic proteins of HEK293 cells. We identified about 1,100 heat-sensitive HEK293 proteins, 12% of which could be isolated in complexes with sHsps. Analysis of their biochemical properties revealed that most of the sHsp substrates have a molecular mass from 50 to 100 kDa and a slightly acidic pI (5.4-6.8). The potency of the sHsps to suppress aggregation of model substrates is correlated with their ability to form stable substrate complexes: especially HspB1 and HspB5 but also B3 bind tightly to a variety of proteins, while less substrates were detected in complex with the other sHsps, although these were also efficient in preventing the aggregation of cytosolic proteins.