The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones
15-Mar-2012
Biochimica et Biophysica Acta, 2012, doi:10.1016/j.bbamcr.2011.09.003, Volume 1823, Issue 3, Pages 624–635 published on 15.03.2012
Biochimica et Biophysica Acta, online article
Biochimica et Biophysica Acta, online article
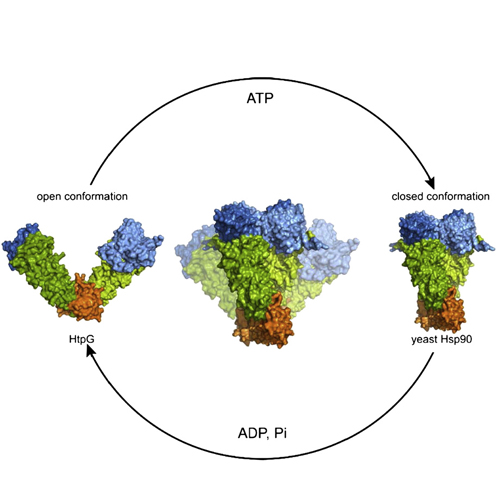
Hsp90 is a dimeric molecular chaperone required for the activation and stabilization of numerous client proteins many of which are involved in essential cellular processes like signal transduction pathways. This activation process is regulated by ATP-induced large conformational changes, co-chaperones and posttranslational modifications. For some co-chaperones, a detailed picture on their structures and functions exists, for others their contributions to the Hsp90 system is still unclear. Recent progress on the conformational dynamics of Hsp90 and how co-chaperones affect the Hsp90 chaperone cycle significantly increased our understanding of the gearings of this complex molecular machinery. This article is part of a Special Issue entitled: Heat Shock Protein 90 (Hsp90).