The HSP90 chaperone machinery
21-Apr-2017
Nature Reviews Molecular Cell Biology, volume 18, 345–360, doi:10.1038/nrm.2017.20
Nature Reviews Molecular Cell Biology, online article
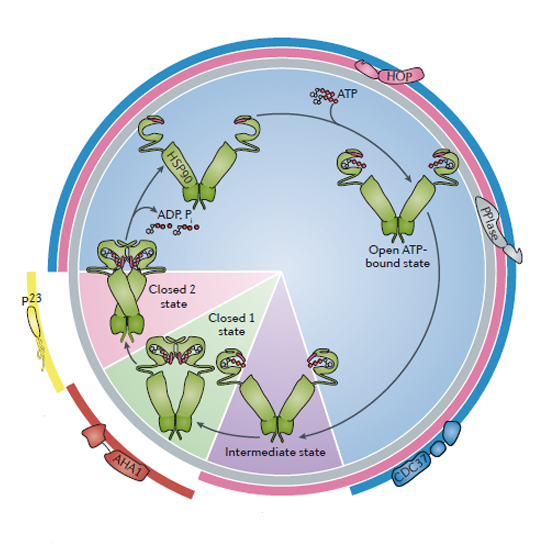
The heat shock protein 90 (HSP90) chaperone machinery is a key regulator of proteostasis under both physiological and stress conditions in eukaryotic cells. As HSP90 has several hundred protein substrates (or 'clients'), it is involved in many cellular processes beyond protein folding, which include DNA repair, development, the immune response and neurodegenerative disease. A large number of co-chaperones interact with HSP90 and regulate the ATPase-associated conformational changes of the HSP90 dimer that occur during the processing of clients. Recent progress has allowed the interactions of clients with HSP90 and its co-chaperones to be defined. Owing to the importance of HSP90 in the regulation of many cellular proteins, it has become a promising drug target for the treatment of several diseases, which include cancer and diseases associated with protein misfolding.