Research Area B - Publications 2008
18-Nov-2008

The TIM23 complex is the major translocase of the mitochondrial inner membrane responsible for the import of essentially all matrix proteins and a number of inner membrane proteins. Tim23 and Tim50, two essential proteins of the complex, expose conserved domains into the intermembrane space which interact with each other. Here, we describe in vitro reconstitution ...
05-Nov-2008

Tic110 has been proposed to be a channel-forming protein at the inner envelope of chloroplasts whose function is essential for the import of proteins synthesized in the cytosol. Sequence features and topology determination experiments presently summarized suggest that Tic110 consists of six transmembrane helices. Its topology has been mapped by limited ...
13-Oct-2008
Angew. Chemie, online article
Proteins are often modified posttranslationally by glycosylation and lipidation. Glycosylphosphatidylinositol (GPI) anchors combine both types of modification and link many proteins to the cell surface. Advances in solid-phase peptide synthesis (SPPS) and recombinant protein engineering, in combination with the development of native chemical ligation (NCL) and ...
14-Aug-2008
PMB, 2008, 68, no. 4-5, 505-19 published on 14.08.2008
Plant Molecular Biology, online article
The chloroplast inner envelope membrane contains many integral proteins which differ in the number of a-helices that anchor the protein into the bilayer. For most of these proteins it is not known which pathway they engage to reach their final localisation within the membrane. In yeast mitochondria, two distinct sorting/insertion pathways have been described for ...
04-Aug-2008
Current Biology, 2008, 18, 15, R639-R641 published on 04.08.2008
Current Biology, online article

What is rheology? Most people are familiar with the basics of rheology from experience with diarrhea or perhaps rheostats. The word rheology was invented in 1929 to name the discipline of a society engaged in the study of how materials deform in response to forces. It was inspired by a quote by Heraclitus: “παντα ρει” translated as “everything flows”. Indeed ...
12-Jul-2008
BBA - Molecular Cell Research, online article
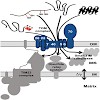
Mitochondria are essential organelles of the eukaryotic cells that are made by expansion and division of pre-existing mitochondria. The majority of their protein constituents are synthesized in the cytosol. They are transported into and put together within the organelle. This complex process is facilitated by several protein translocases. Here we summarize ...
09-Jul-2008

Actin belongs to the most abundant proteins in eukaryotic cells which harbor usually many conventional actin isoforms as well as actin-related proteins (Arps). To get an overview over the sometimes confusing multitude of actins and Arps, we analyzed the Dictyostelium discoideum actinome in detail and compared it with the genomes from other model organisms. The D. ...
02-Jul-2008

Folding intermediates play a key role in defining protein folding and assembly pathways as well as those of misfolding and aggregation. Yet, due to their transient nature, they are poorly accessible to high-resolution techniques. Here, we made use of the intrinsically slow folding reaction of an antibody domain to characterize its major folding intermediate in ...
31-May-2008
Journal of the American Chemical Society, 2008, 130, 8079-84 published on 31.05.2008
JACS, online article

Thioxoamide (thioamide) bonds are nearly isosteric substitutions for amides but have altered hydrogen-bonding and photophysical properties. They are thus well-suited backbone modifications for physicochemical studies on peptides and proteins. The effect of thioxoamides on protein structure and stability has not been subject to detailed experimental investigations ...
21-May-2008
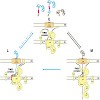
The TIM23 (translocase of the mitochondrial inner membrane) complex mediates translocation of preproteins across and their insertion into the mitochondrial inner membrane. How the translocase mediates sorting of preproteins into the two different subcompartments is poorly understood. In particular, it is not clear whether association of two operationally defined ...
07-May-2008
Journal of Bacteriology, online article
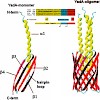
The Oca family is a novel class of autotransporter-adhesins with highest structural similarity in their C-terminal transmembrane region, which supposedly builds a beta-barrel pore in the outer membrane (OM). The prototype of the Oca family is YadA, an adhesin of Yersinia enterocolitica and Yersinia pseudotuberculosis. YadA forms a homotrimeric lollipop-like ...
26-Apr-2008
Histochemistry; online article

In this article, we follow the history of one of the most abundant, most intensely studied proteins of the eukaryotic cells: actin. We report on hallmarks of its discovery, its structural and functional characterization and localization over time, and point to present days’ knowledge on its position as a member of a large family. We focus on the rather puzzling ...
12-Apr-2008
Biochimica et Biophysica Acta, online article
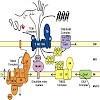
Biogenesis of mitochondria depends on the coordinated action of at least six protein translocases present in both mitochondrial membranes. They use different energy sources to drive unidirectional transport of proteins across and into mitochondrial membranes. Here we present an overview on the energetic requirements of different mitochondrial import pathways.
04-Apr-2008
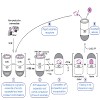
The GroEL/GroES chaperonin system mediates protein folding in the bacterial cytosol. Newly synthesized proteins reach GroEL via transfer from upstream chaperones such as DnaK/DnaJ (Hsp70).Here we employed single molecule and ensemble FRET to monitor the conformational transitions of amodel substrate as it proceeds along this chaperone pathway. We find that ...
04-Apr-2008
Journal of Bacteriology, online article

As a first approach to establishing a three-dimensional culture infection model, we studied the growth behavior of the extracellular pathogen Yersinia enterocolitica in three-dimensional collagen gels (3D-CoG). Surprisingly, we observed that plasmidless Y. enterocolitica was motile in the 3D-CoG in contrast to its growth in traditional motility agar at 37°C. ...
14-Mar-2008
The Jounal of Biological Chemistry, 2008, 283, 6656-67 published on 14.03.2008
www.jbc.org, online article

The preprotein translocon at the inner envelope of chloroplasts (Tic complex) facilitates the import of nuclear-encoded preproteins into the organelle. Seven distinct subunits have been identified so far. For each of those, specific functions have been proposed based on structural prediction or experimental evidence. Three of those subunits possess modules that ...
12-Feb-2008

Small heat shock proteins (sHsps) are a widespread and diverse class of molecular chaperones. In vivo, sHsps contribute to thermotolerance. Recent evidence suggests that their function in the cellular chaperone network is to maintain protein omeostasis by complexing a variety of non-native proteins. One of the most characteristic features of sHsps is their ...
01-Feb-2008

Cells respond to a sudden increase in temperature with the transcription of a special set of genes, a phenomenon known as the heat shock response. In the yeast S. cerevisiae, the molecular chaperone Hsp26 is one component of the heat shock response. Hsp26 has the remarkable ability to sense increases in temperature directly and can switch from an inactive to a ...