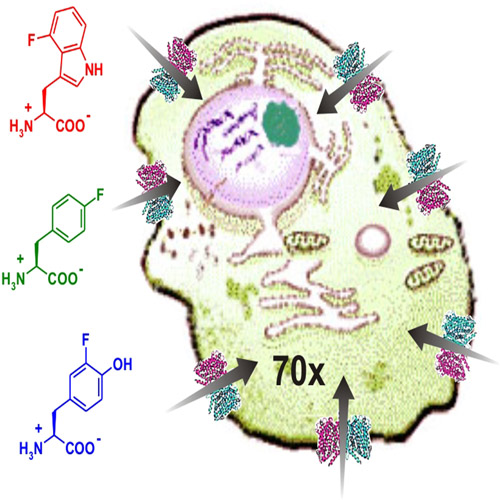
Intracellular uptake and inhibitory activity of aromatic fluorinated amino acids in human breast cancer cells
05-Sep-2008
Nonproteinogenic amino acids that either occur naturally or are synthesized chemically are becoming important tools in modern drug discovery. In this context, fluorinated amino acids have great potential in the development of novel pharmaceuticals and drugs. To assess whether different fluorinated aromatic amino acid analogues of phenylalanine, tyrosine, and tryptophan are potentially interesting as therapeutic drugs, we examined their cytostatic and cytotoxic effects on the growth of the human breast cancer cell line MCF-7. Of all the tested analogues l-4-fluorotryptophan, l-6-fluorotryptophan and l-p-fluorophenylalanine effectively and irreversibly inhibited cell growth with IC50 values in the low micromolar range (3–15 mm). Additionally, using l-4- [14C]fluorotryptophan, and l-6-[14C]fluorotryptophan, we discovered that the cellular uptake of these fluorinated amino acids occurs through active transport with a 70-fold excess of intracellular over extracellular concentrations. We identified system L as the responsible amino acid transporter. Our findings fully support the idea that fluorinated aromatic amino acid analogues are promising chemotherapeutics with the potential for use in combination with classical cancer therapy, and as new cytotoxic drugs for certain tumor types such as melanoma.