Role of the cytosolic loop C2 and the C-terminus of YidC in ribosome binding and insertion activity
28-May-2015
THE JOURNAL OF BIOLOGICAL CHEMISTRY, VOL. 290, NO. 28, pp. 17250–17261, DOI 10.1074/jbc.M115.650309
THE JOURNAL OF BIOLOGICAL CHEMISTRY, online article
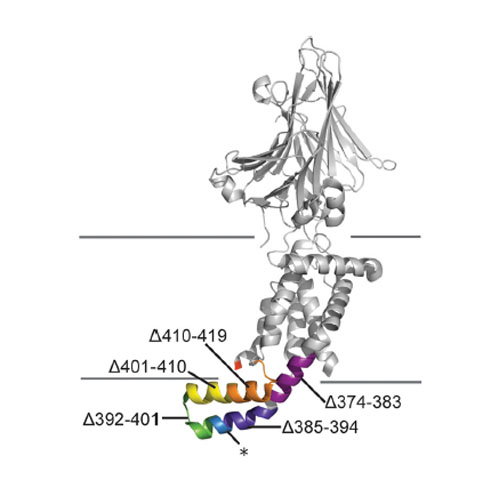
Members of the YidC/Oxa1/Alb3 protein family mediate membrane protein insertion and this process is initiated by the assembly of YidC:ribosome nascent chain (RNC) complexes at the inner leaflet of the lipid bilayer. The positively charged C-terminus of Escherichia coli YidC plays a significant role in ribosome binding but is not the sole determinant as deletion does not completely abrogate ribosome binding. The positively charged cytosolic loops C1 and C2 of YidC may provide additional docking sites. We performed systematic sequential deletions within these cytosolic domains and studied their effect on the YidC insertase activity and interaction with translation-stalled (programmed) ribosome. Deletions within loop C1 strongly affected the activity of YidC in vivo, but did not influence ribosome binding or substrate insertion, while loop C2 appeared to be involved in ribosome binding. Combining the latter deletion with the removal of the C-terminus of YidC abolished YidC-mediated insertion. We propose that these two regions play an crucial role in the formation and stabilization of an active YidC:RNC complex, allowing for co-translational membrane insertion, while loop C1 may be involved in the downstream chaperone activity of YidC or in other protein:protein interactions.