The Antibody Light-Chain Linker Is Important for Domain Stability and Amyloid Formation
06-Nov-2015
Journal of Molecular Biology, Volume 427, Issue 22, Pages 3572–3586, doi:10.1016/j.jmb.2015.09.012
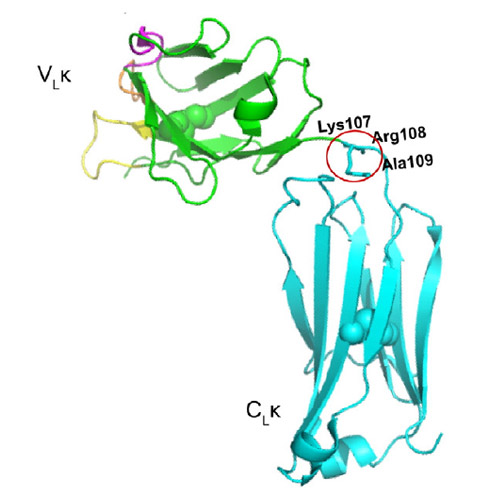
The association of light chains (LCs) and heavy chains is the basis for functional antibodies that are essential for adaptive immune responses. However, in some cases, LCs and especially fragments consisting of the LC variable (VL) domain are pathologically deposited in fatal aggregation diseases. The two domains of the LC are connected by a highly conserved linker. We show here that, unexpectedly, the linker residue Arg108 affects the conformational stability and folding of both VLκ and LC constant (CLκ) domains. Interestingly, the extension of VL by Arg108 results in its resistance to amyloid formation, which suggests that the nature of the truncation of the LC plays a crucial role in disease progression. Increased solvation due to the exposed charged C-terminal Arg108 residue explains its stabilizing effects on the VL domain. For the CL domain, the interaction of N-terminal loop residues with Arg108 is important for the integrity of the domain, as the disruption of this interaction results in fluctuation, partial opening of the protein's interior and the exposure of hydrophobic residues that destabilize the domain. This establishes new principles for antibody domain architecture and amyloidogenicity.