20S Proteasome Inhibition: Designing Noncovalent Linear Peptide Mimics of the Natural Product TMC-95A
16-Aug-2010
ChemMedChem,, 2010, DOI: 10.1002/cmdc.201000293, Volume 5, Issue 10, pages 1701–1705 published on 16.08.2010
ChemMedChem, online article
ChemMedChem, online article
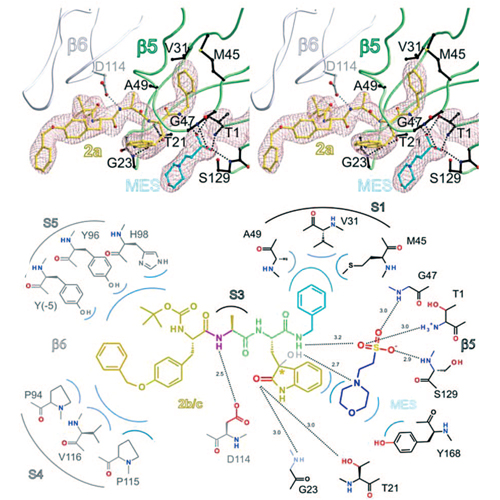
The ubiquitin-proteasome system (UPS) is responsible for the controlled degradation of proteins in eukaryotic cells through conjugation of ubiquitin molecules and subsequent cleavage by a multimeric protein complex known as the 26S proteasome. Proteolysis occurs within the 20S proteasome core particle (CP), a large barrel-shaped protein complex composed of four stacked rings made up of a and b subunits and which is found throughout all the three kingdoms of life. The a subunits constitute the top and bottom of the four rings and have substrate gating functions, whereas the b subunits, found within the two central rings of the complex, supply the catalytic machinery. In eukaryotes, this CP is composed of seven unique a subunits and seven unique b subunits, of which only three—b1, b2, and b5—possess a catalytic N-terminal threonine residue required for substrate hydrolysis (with chymotrypsin- like (CL), trypsin-like (TL), and peptidyl-glutamyl-peptide-hydrolyzing (PGPH) activities, respectively; see Supporting Information comment SC1).