A Genetically Encoded Norbornene Amino Acid for the Mild and Selective Modification of Proteins in a Copper-Free Click Reaction
27-Apr-2012
Angewandte Chemie, 2012, DOI: 10.1002/anie.201109252, Volume 51, Issue 18, pages 4466–4469 published on 27.04.2012
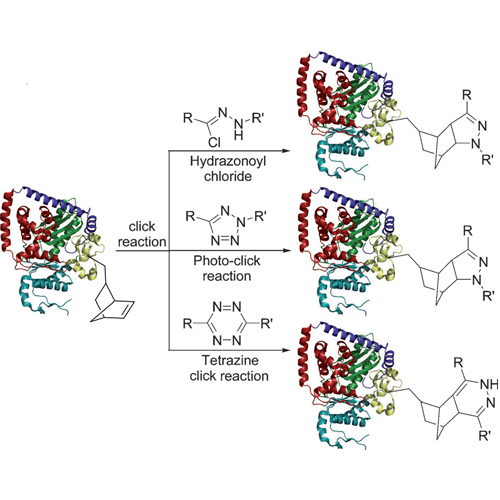
Methods for the site-specific chemical modification of proteins are currently of immense importance for the synthesis of protein–hybrid compounds for pharmaceutical and diagnostic purposes. Most of the methods rely on the reaction of free protein thiols with maleimides or the reaction of lysine side chains with activated esters. These methods provide only limited specificity, which is prompting researchers to develop alternative strategies that involve the incorporation of special unnatural amino acid into proteins to enable site-specific bioorthogonal functionalization. Among the developed methods, the CuI-catalyzed reaction of a protein containing an alkyne amino acid with azides stands out as the most thoroughly investigated technology. However, the need for CuI salts, which may harm the protein structure, limits the technology. This fuels current interest to develop copperfree coupling reactions that are compatible with fragile protein structures. Here we show that these requirments can be met with a specially encoded norbornene amino acid which reacts selectively with nitrile imines.