AAA+ chaperones and acyldepsipeptides activate the ClpP protease via conformational control
19-Feb-2015
Nature Communications, 2015, doi:10.1038/ncomms7320, 6, Article number: 6320 published on 19.02.2015
Nature Communications, online article
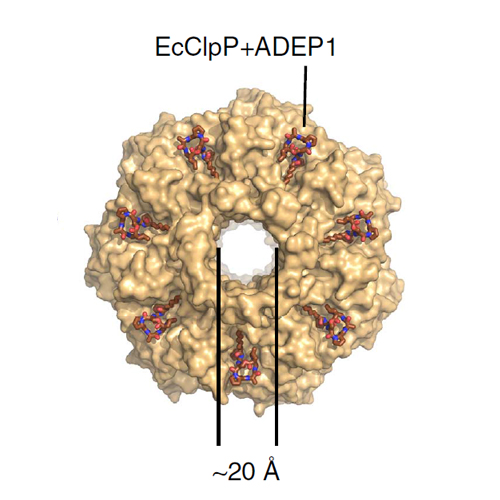
The Clp protease complex degrades a multitude of substrates, which are engaged by a AAA+ chaperone such as ClpX and subsequently digested by the dynamic, barrel-shaped ClpP protease. Acyldepsipeptides (ADEPs) are natural product-derived antibiotics that activate ClpP for chaperone-independent protein digestion. Here we show that both protein and small-molecule activators of ClpP allosterically control the ClpP barrel conformation. We dissect the catalytic mechanism with chemical probes and show that ADEP in addition to opening the axial pore directly stimulates ClpP activity through cooperative binding. ClpP activation thus reaches beyond active site accessibility and also involves conformational control of the catalytic residues. Moreover, we demonstrate that substoichiometric amounts of ADEP potently prevent binding of ClpX to ClpP and, at the same time, partially inhibit ClpP through conformational perturbance. Collectively, our results establish the hydrophobic binding pocket as a major conformational regulatory site with implications for both ClpXP proteolysis and ADEP-based anti-bacterial activity.