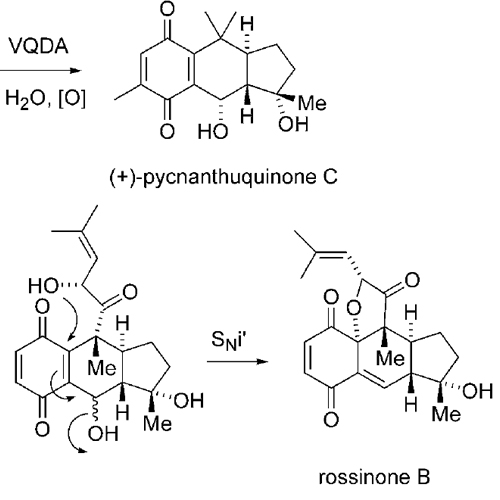
Biomimetic Synthesis of (−)-Pycnanthuquinone C through the Diels–Alder Reaction of a Vinyl Quinone
19-Jul-2010
Angewandte Chemie, 2010, 49, 35, 6199 - 6202 published on 19.07.2010
Angewandte Chemie, online article
Diels–Alder reactions of vinyl quinones may provide a rapid entry to highly functionalized bi- and polycyclic ring systems. They involve the inverse-electron-demand cycloaddition of a suitable dienophile to a vinyl quinone, which presumably generates an “isoquinone methide” (Scheme 1). This reactive intermediate could then tautomerize in several ways to yield quinone methides, bicyclic quinones, or hydroquinones. If the isoquinone methide or quinone methide is intercepted by a nucleophile, such as water, a functionalized tetraline hydroquinone may result. This may be oxidized readily to the functionalized tetraline quinone.