Convenient Access to Di- and Trifluoroethylamines for Lead Structure Research
03-Feb-2016
Eur. J. Org. Chem., Volume 2016, Issue 5, Pages 930–945, DOI: 10.1002/ejoc.201501576
Eur. J. Org. Chem., online article
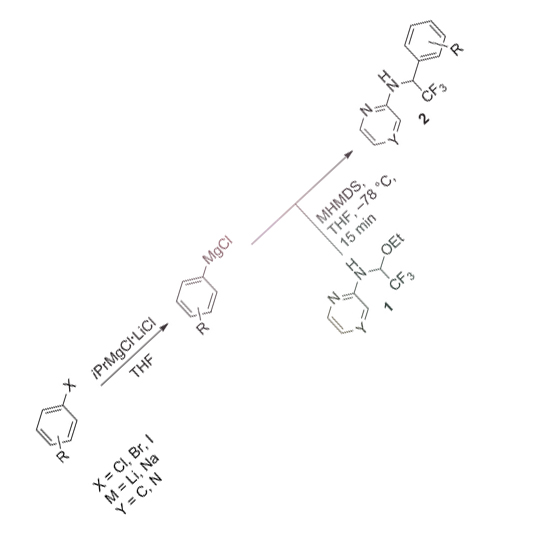
Much research effort has been devoted to the synthesis of fluorinated organic compounds in medicinal chemistry programs. For instance, incorporation of fluorine substituents and trifluoromethyl groups has become a widespread lead optimization strategy owing to the often favorable influence of such moieties on affinity and physicochemical properties. However, introduction of fluoroalkyl groups into dedicated positions of pharmacophores is synthetically challenging. In particular, efficient syntheses of di- and trifluoroethylamines are needed as they are an interesting, yet underrepresented, class of carboxamide mimics. Thus, a practical and reliable protocol for their preparation is described by using functionalized di- and trifluoromethylated N-aryl-substituted N,O-acetals as synthetic aldimine equivalents. Moreover, previously unknown β-trifluoroethylamine analogs of 1,2-dihydroquinazolin-4(1H)-thione and the androgen receptor antagonist DIMN are prepared to demonstrate the method's applicability.