Crystal structure of the human odorant binding protein, OBPIIa
04-Apr-2015
Proteins, Volume 83, Issue 6, Pages 1180–1184, DOI: 10.1002/prot.24797
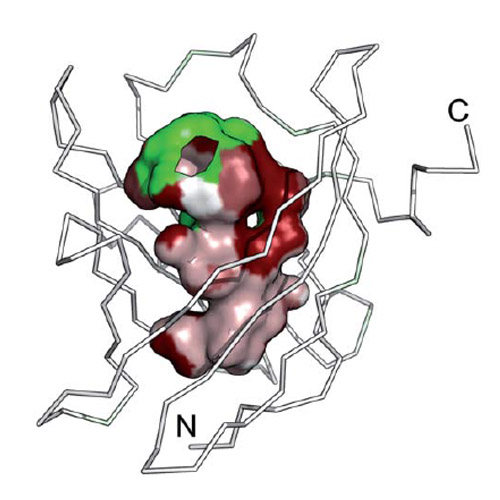
Human odorant-binding protein, OBPIIa, is expressed by nasal epithelia to facilitate transport of hydrophobic odorant molecules across the aqueous mucus. Here, we report its crystallographic analysis at 2.6 Å resolution. OBPIIa is a monomeric protein that exhibits the classical lipocalin fold with a conserved eight-stranded β-barrel harboring a remarkably large hydrophobic pocket. Basic residues within the four loops that shape the entrance to this ligand-binding site evoke a positive electrostatic potential. Human OBPIIa shows distinct features compared with other mammalian OBPs, including a potentially reactive Cys side chain within its pocket similar to human tear lipocalin.