Efficient N-Terminal Glycoconjugation of Proteins by the N-End Rule
02-Apr-2008
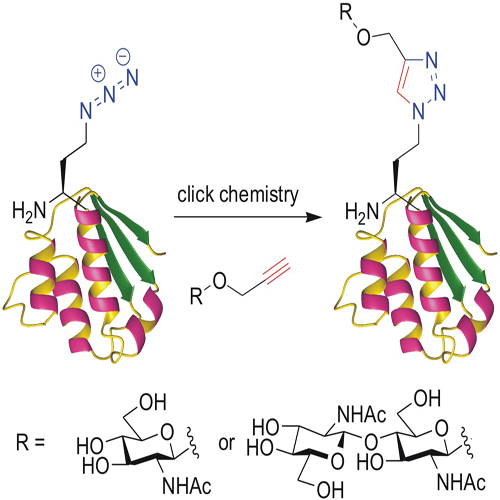
The importance of protein N terminus sequence composition for cell physiology was recognized more than two decades ago. However, its relevance for chemical protein engineering through an expanded genetic code was demonstrated only very recently. Nature changes the chemistry of the N terminus by posttranslational modifications (PTMs) such as longchain alkylation, acetylation, myristoylation, glycosylation, etc. This, in turn, influences the lifetimes and general metabolic fates of tagged proteins in different ways according to the N-end rules. Although Met is the first amino acid in a newly synthesized protein, it is usually enzymatically removed from the mature protein when the second position is occupied by a non-bulky residue (for example, Ala, Cys, Gly). On the other hand, bulky amino acids—such as Lys, Arg, Leu, Phe, and Ile—in this position protect the N-terminal Met from being processed. The extension of these rules to noncanonical amino acids occupying the protein N terminus should enable the in vivo generation of stable artificial N-terminal handles. Their subsequent chemical derivatization might generate new specific functions, especially if carbohydrates are attached.