Engineering of alanine dehydrogenase from Bacillus subtilis for novel cofactor specificity
11-Sep-2015
Biotechnology and Applied Biochemistry, Volume 63, Issue 5, Pages 616–624, DOI: 10.1002/bab.1414
Biotechnology and Applied Biochemistry, online article
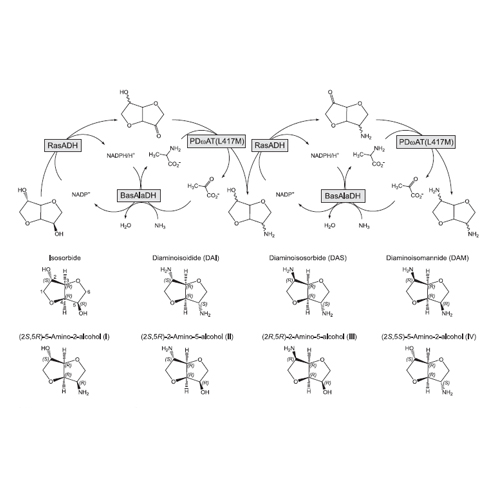
The l-alanine dehydrogenase of Bacillus subtilis (BasAlaDH), which is strictly dependent on NADH as redox cofactor, efficiently catalyzes the reductive amination of pyruvate to l-alanine using ammonia as amino group donor. To enable application of BasAlaDH as regenerating enzyme in coupled reactions with NADPH-dependent alcohol dehydrogenases, we alterated its cofactor specificity from NADH to NADPH via protein engineering. By introducing two amino acid exchanges, D196A and L197R, high catalytic efficiency for NADPH was achieved, with kcat/KM = 54.1 µM−1 Min−1 (KM = 32 ± 3 µM; kcat = 1,730 ± 39 Min−1), almost the same as the wild-type enzyme for NADH (kcat/KM = 59.9 µM−1 Min−1; KM = 14 ± 2 µM; kcat = 838 ± 21 Min−1). Conversely, recognition of NADH was much diminished in the mutated enzyme (kcat/KM = 3 µM−1 Min−1). BasAlaDH(D196A/L197R) was applied in a coupled oxidation/transamination reaction of the chiral dicyclic dialcohol isosorbide to its diamines, catalyzed by Ralstonia sp. alcohol dehydrogenase and Paracoccus denitrificans ω-aminotransferase, thus allowing recycling of the two cosubstrates NADP+ and l-Ala. An excellent cofactor regeneration with recycling factors of 33 for NADP+ and 13 for l-Ala was observed with the engineered BasAlaDH in a small-scale biocatalysis experiment. This opens a biocatalytic route to novel building blocks for industrial high-performance polymers.