Evaluation of Kinase Activity Profiling Using Chemical Proteomics
17-Sep-2015
ACS Chem. Biol., 10 (12), pp 2743–2752, DOI: 10.1021/acschembio.5b00616
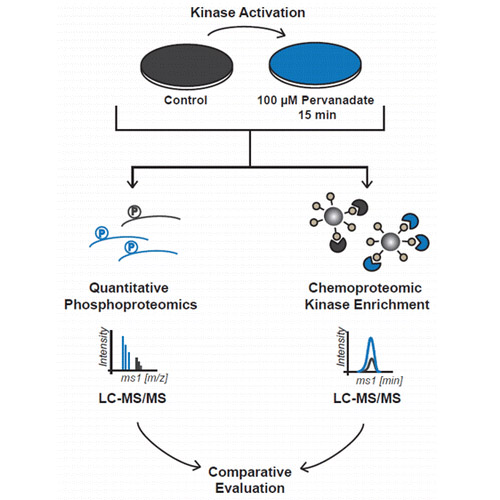
Protein kinases are important mediators of intracellular signaling and are reversibly activated by phosphorylation. Immobilized kinase inhibitors can be used to enrich these often low-abundance proteins, to identify targets of kinase inhibitors, or to probe their selectivity. It has been suggested that the binding of kinases to affinity beads reflects a kinase’s activation status, a concept that is under considerable debate. To assess the merits of the idea, we performed a series of experiments including quantitative phosphoproteomics and purification of kinases by single or mixed affinity matrices from signaling activated or resting cancer cells. The data show that mixed affinity beads largely bind kinases independent of their activation status, and experiments using individual immobilized kinase inhibitors show mixed results in terms of preference for binding the active or inactive conformation. Taken together, activity- or conformation-dependent binding to such affinity resins depends (i) on the kinase, (ii) on the affinity probe, and (iii) on the activation status of the lysate or cell. As a result, great caution should be exercised when inferring kinase activity from such binding data. The results also suggest that assaying kinase activity using binding data is restricted to a limited number of well-chosen cases.