Kinetic and Theoretical Studies of Beta-Lactone Reactivity—A Quantitative Scale for Biological Application
16-Jul-2015
ChemPlusChem, Volume 80, Issue 11, pages 1673–1679, DOI: 10.1002/cplu.201500246
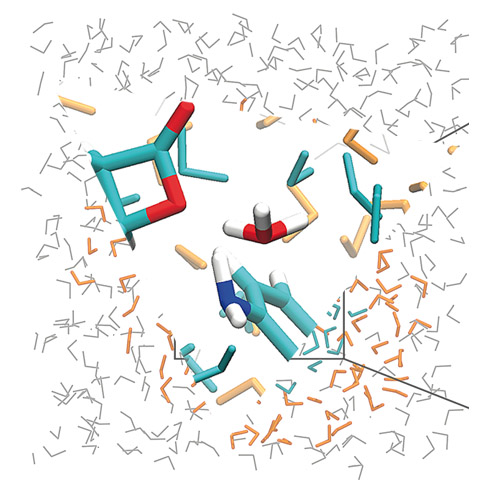
Natural products comprise a rich source for bioactive molecules with medicinal relevance. Many of these contain electrophilic scaffolds that bind conserved enzyme active sites covalently. Prominent examples include beta-lactams and beta-lactones, which specifically acylate serine residues in diverse peptidases. Although these scaffolds appear similar, their bioactivities and corresponding protein targets vary. To quantify and dissect these differences in bioactivities, the kinetics of the reactions of beta-butyrolactone with a set of reference amines in buffered aqueous solution at 37 °C have been analyzed. Different product ratios of C1 versus C3 attack on the beta-butyrolactone have been observed, depending on the aliphatic or aromatic nature of the standard amine used. Quantum mechanics/molecular mechanics (QM/MM) calculations reveal that a H3O+ molecule has a crucial role in stabilizing C3 attack by aniline, through coordination of the lactone ring oxygen. In agreement with their weak proteome reactivity, monocyclic beta-lactams did not react with the set of standard nucleophiles studied herein. Bicyclic beta-lactams, however, exhibited a lower activation barrier, and thus, reacted with standard nucleophiles. This study represents a starting point for semiquantitative reactivity scales for natural products, which, in analogy to chemical reactivity scales, will provide predictions for electrophilic modifications in biological systems.