Unraveling the Protein Targets of Vancomycin in Living S. aureus and E. faecalis Cells
07-Jul-2011
Jacs, 2011, DOI: 10.1021/ja2039979, 133 (31), pp 12144–12153 published on 07.07.2011
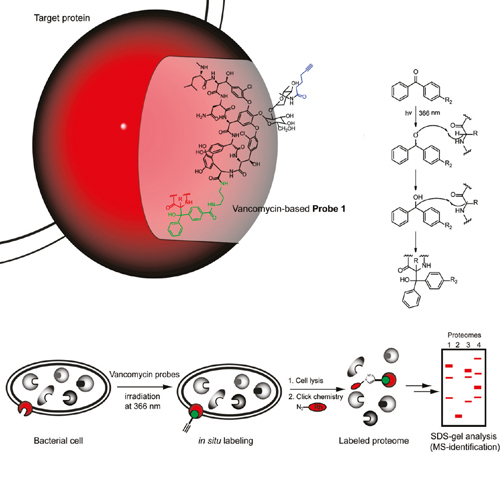
Vancomycin is a potent glycopeptide antibiotic that has evolved to specifically bind to the D-Ala-D-Ala dipeptide termini of nascent peptidoglycans. Although this mode of action is well established, several studies indicate that vancomycin and analogues exploit noncanonical target sites. In order to address all vancomycin targets in clinically relevant Staphylococcus aureus and Enterococcus faecalis strains we developed a series of small-molecule photoaffinity probes based on vancomycin. Proteomic profiling revealed the specific labeling of two previously unknown vancomycin targets that are likely to contribute to its antibiotic activity. The specific inhibition of the major staphylococcal autolysin Atl confirms previous observations that vancomycin alters S. aureus cell morphology by interaction with the autolytic machinery. Moreover, in E. faecalis the vancomycin photoprobe specifically binds to an ABC transporter protein, which likely impedes the uptake of essential nutrients such as sugars and peptides. The labeling of these two prominent membrane targets in living cells reveals a thus far unexplored mode of vancomycin binding and inhibition that could allow a rational design of variants with improved activity.