Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells
01-Sep-2015
Cell Cycle, Volume 14 Issue 17, 2810--2820; http://dx.doi.org/10.1080/15384101.2015.1068482
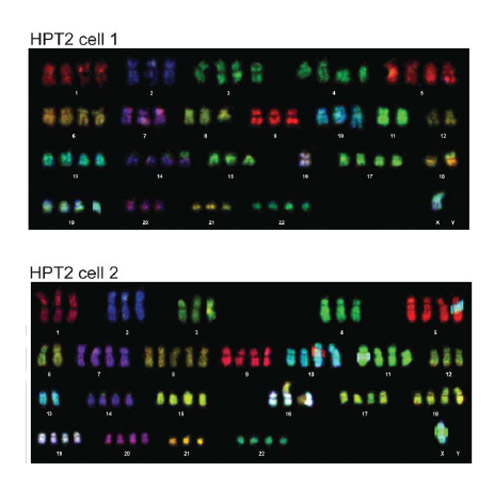
Up to 80% of human cancers, in particular solid tumors, contain cells with abnormal chromosomal numbers, or aneuploidy, which is often linked with marked chromosomal instability. Whereas in some tumors the aneuploidy occurs by missegregation of one or a few chromosomes, aneuploidy can also arise during proliferation of inherently unstable tetraploid cells generated by whole genome doubling from diploid cells. Recent findings from cancer genome sequencing projects suggest that nearly 40% of tumors underwent whole genome doubling at some point of tumorigenesis, yet its contribution to cancer phenotypes and benefits for malignant growth remain unclear. Here, we investigated the consequences of a whole genome doubling in both cancerous and non-transformed p53 positive human cells. SNP array analysis and multicolor karyotyping revealed that induced whole-genome doubling led to variable aneuploidy. We found that chromosomal instability (CIN) is a frequent, but not a default outcome of whole genome doubling. The CIN phenotypes were accompanied by increased tolerance to mitotic errors that was mediated by suppression of the p53 signaling. Additionally, the expression of pro-apoptotic factors, such as iASPP and cIAP2, was downregulated. Furthermore, we found that whole genome doubling promotes resistance to a broad spectrum of chemotherapeutic drugs and stimulates anchorage-independent growth even in non-transformed p53-positive human cells. Taken together, whole genome doubling provides multifaceted benefits for malignant growth. Our findings provide new insight why genome-doubling promotes tumorigenesis and correlates with poor survival in cancer.