CTD Tyrosine Phosphorylation Impairs Termination Factor Recruitment to RNA Polymerase II
29-Jun-2012
Science, 2012, DOI: 10.1126/science.1219651, Vol. 336 no. 6089 pp. 1723-1725 published on 29.06.2012
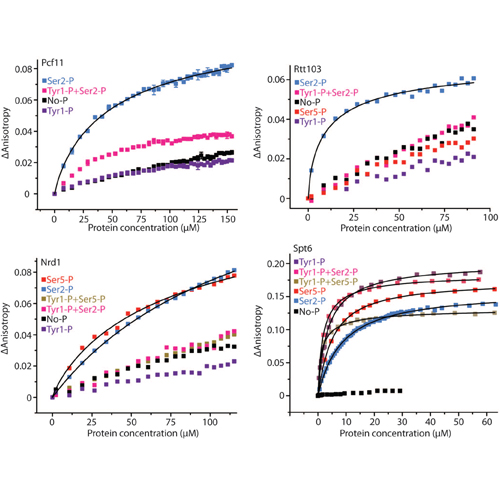
In different phases of the transcription cycle, RNA polymerase (Pol) II recruits various factors via its C-terminal domain (CTD), which consists of conserved heptapeptide repeats with the sequence Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7. We show that the CTD of transcribing yeast Pol II is phosphorylated at Tyr1, in addition to Ser2, Thr4, Ser5, and Ser7. Tyr1 phosphorylation stimulates binding of elongation factor Spt6 and impairs recruitment of termination factors Nrd1, Pcf11, and Rtt103. Tyr1 phosphorylation levels rise downstream of the transcription start site and decrease before the polyadenylation site, largely excluding termination factors from gene bodies. These results show that CTD modifications trigger and block factor recruitment and lead to an extended CTD code that explains transcription cycle coordination on the basis of differential phosphorylation of Tyr1, Ser2, and Ser5.