Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes
20-Oct-2008
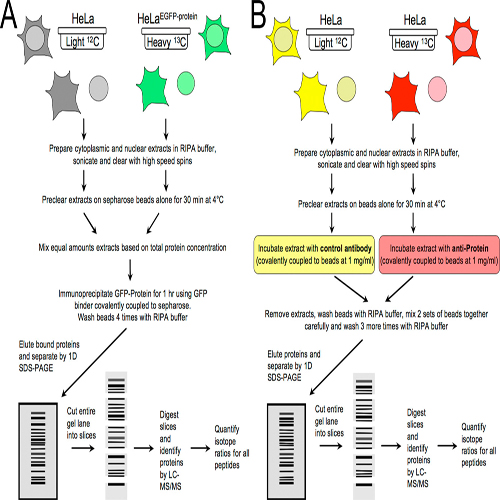
The identification of interaction partners in protein complexes is a major goal in cell biology. Here we present a reliable affi nity purifi cation strategy to identify specifi c interactors that combines quantitative SILAC-based mass spectrometry with characterization of common contaminants binding to affi nity matrices (bead proteomes). This strategy can be applied to affi nity purifi cation of either tagged fusion protein complexes or endogenous protein complexes, illustrated here using the well-characterized SMN complex as a model. GFP is used as the tag of choice because it shows minimal nonspecific binding to mammalian cell proteins, can be quantitatively depleted from cell extracts, and allows the integration of biochemical protein interaction data with in vivo measurements using fl uorescence microscopy. Proteins binding nonspecifi cally to the most commonly used affi nity matrices were determined using quantitative mass spectrometry, revealing important differences that affect experimental design. These data provide a specificity filter to distinguish specific protein binding partners in both quantitative and and nonquantitative pull-down and immunoprecipitation experiments.