Phosphorylation by cyclin-dependent kinase-9 controls ubiquitin-conjugating enzyme-2A function
01-Jun-2012
Cell Cycle, 2012, http://dx.doi.org/10.4161/cc.20548, Volume 11, Issue 11, 2122 - 2127 published on 01.06.2012
Cell Cycle, online article
Cell Cycle, online article
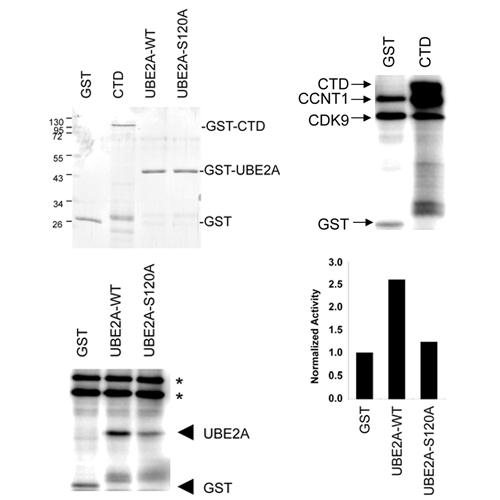
Cyclin-dependent kinase-9 (CDK9) plays a central role in transcriptional elongation and controls multiple cotranscriptional histone modifications, including histone H2B monoubiquitination (H2Bub1). Like other CDK9-dependent histone modifications, the role of CDK9 in maintaining H2Bub1 was shown to be partially dependent upon the phosphorylation status of Ser2 of the RNA polymerase II (RNAPII) C-terminal domain (CTD). Since mutation of Ser2 within the RNAPII CTD resulted in a milder effect on H2Bub1 compared with CDK9 knockdown, we explored whether another CDK9 target may also influence H2Bub1. Based on its homology to yeast Bur1, we hypothesized that CDK9 may directly phosphorylate and activate the ubiquitin-conjugating enzyme utilized for H2B monoubiquitination. Indeed, we demonstrate that UBE2A specifically interacts with CDK9, but not CDK2. Furthermore, UBE2A is phosphorylated by CDK9 in vitro and increases UBE2A activity. Interestingly, CDK9 knockdown not only decreases UBE2A phosphorylation and H2Bub1, but also significantly impairs the induction of UBE2A-dependent monoubiquitination of proliferating cell nuclear antigen (PCNA). Thus, we provide the first evidence that CDK9 is required for the activity of UBE2A in humans, and that its activity is not only required for maintaining H2Bub1, but also for the monoubiquitination of PCNA. The common involvement of these two ubiquitinations in distinct DNA repair pathways may provide a mechanistic rationale for further exploring CDK9 as a combinatorial target for increasing the efficacy of existing cancer therapies based on the induction of DNA damage and are repaired by mechanisms which require H2Bub1 and/or PCNA ubiquitination.