Stimulation of the potassium sensor KdpD kinase activity by interaction with the phosphotransferase protein IIANtr in Escherichia coli
29-Apr-2009
Molecular Microbiology, 2009, 72 Issue 4, 978-94 published on 29.04.2009
Molecular Microbiology, online article
Molecular Microbiology, online article
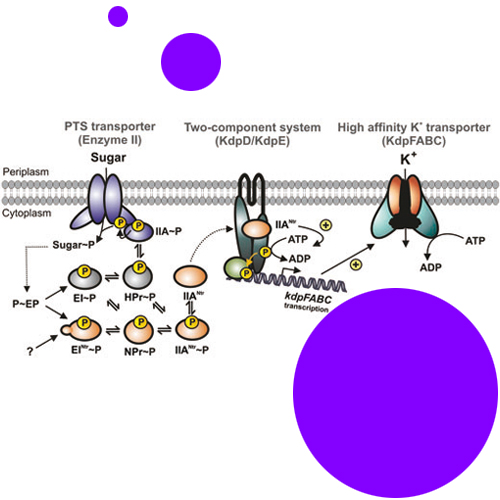
Proteins EINtr, NPr and IIANtr form a phosphoryl group transfer chain (Ntr-PTS) working in parallel to the phosphoenolpyruvate:carbohydrate phosphotransferase system (transport-PTS) in Escherichia coli. Recently, it was shown that dephosphorylated IIANtr binds and inhibits TrkA, a low-affinity potassium transporter. Here we report that the Ntr-PTS also regulates expression of the high-affinity K+ transporter KdpFABC, which rescues K+ uptake at limiting K+ concentrations. Transcription initiation at the kdpFABC promoter is positively controlled by the two-component system KdpD/KdpE in response to K+ availability. We found that kdp promoter activity is stimulated by the dephosphorylated form of IIANtr. Two-hybrid data and biochemical analysis revealed that IIANtr interacts with sensor kinase KdpD and stimulates kinase activity, resulting in increased levels of phosphorylated response regulator KdpE. The data suggest that exclusively dephosphorylated IIANtr binds and activates KdpD. As there is cross-talk between the Ntr-PTS and the transport-PTS, carbon source utilization affects kdpFABC expression. Expression is enhanced, when cells utilize preferred carbohydrates like glucose, which results in preferential dephosphorylation of the transport-PTS and also of IIANtr. Taken together, the data show that the Ntr-PTS has an important role in maintaining K+ homeostasis and links K+ uptake to carbohydrate metabolism.