Biomimetic Total Synthesis of Santalin Y
20-Apr-2015
Angewandte Chemie International Edition, 2015, DOI: 10.1002/anie.201411350, Volume 54, Issue 17, pages 5079–5083, published on 20.04.2015
Angewandte Chemie International Edition, online article
Angewandte Chemie International Edition, online article
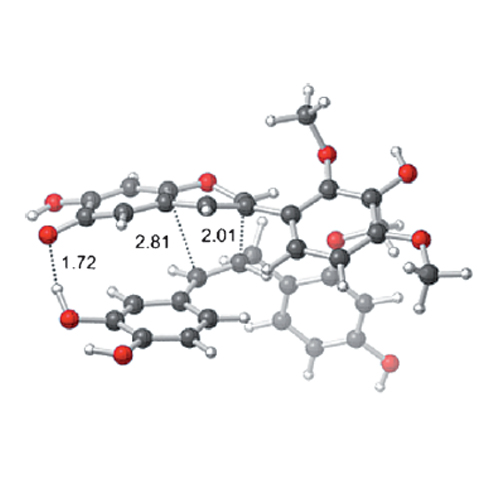
A biomimetic total synthesis of santalin Y, a structurally complex but racemic natural product, is described. The key step is proposed to be a (3+2) cycloaddition of a benzylstyrene to a “vinylogous oxidopyrylium”, which is followed by an intramolecular Friedel–Crafts reaction. This cascade generates the unique oxafenestrane framework of the target molecule and sets its five stereocenters in one operation. Our work provides rapid access to santalin Y and clarifies its biosynthetic relationship with other colorants isolated from red sandalwood.