Endoplasmic reticulum retetion of the gamma-secretase complex component Pen2 by Rer1
06-Jul-2007
EMBO reports, 2007, 1-6 (pdf) published on 06.07.2007
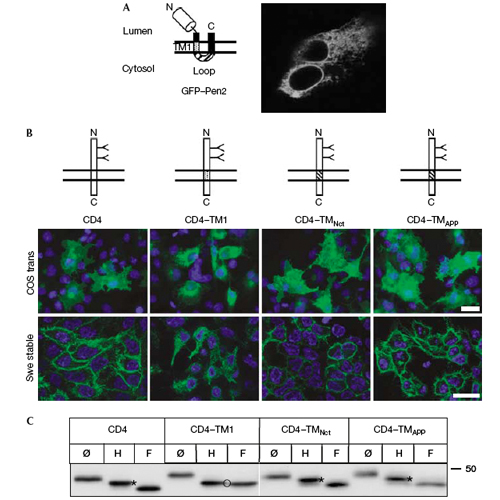
Gamma-Secretase is involved in the production of amyloid beta-peptide, which is the principle component of amyloid plaques in the brains of patients with Alzheimer disease. Gamma-Secretase is a complex composed of presenilin (PS), nicastrin, anterior pharynx-defective phenotype 1 (Aph1) and PS enhancer 2 (Pen2). We previously proposed a mechanism of complex assembly by which unassembled subunits are retained in the endoplasmic reticulum (ER) and only the fully assembled complex is exported from the ER. We have now identified Retention in endoplasmic reticulum 1 (Rer1) as a protein that is involved in the retention/retrievel of unassembled Pen2 to the ER. Direct binding of unassembled Pen2 to Rer1 is mediated by the first transmembrane domain of Pen2, and a conversed asparagine in this domain is required. Downregualtion of Rer1 leads to increased surface localization of Pen2, whereas overexpression of Rer1 stabilizes unassembled Pen2. To our knowledge, Per1 is the first identified interaction partner of mammalian transmembrane-based retention/retrieval signals.